History of the plasma lamp
The first works of physicists on this principle date back to the United States in the 1970s. Sulfur was quickly identified as a visible radiation emitter with very high energy efficiency. The conversion between radio frequency and visible radiation is 170 lm/W. It consists of a sealed glass tube with two metal electrodes, usually tungsten, mounted at each end. The gas inside the tube is usually argon or neon. When electricity is applied to the electrodes, a discharge occurs and the gas becomes ionized, producing a glowing plasma.The first plasma lamps on the market were UV lamps for disinfection, marketed by UV-Fusion.
In the 1990s, an American company called Fusion Lighting was created to popularize this principle. They developed an industrial lighting device (1000 W lamp) to light halls or warehouses.
In the 2000's, other companies improve the equipment to continue the adventure of plasma lamps. New models with very specific characteristics appear on the market. The plasma lamp, for example, has become the best device for faithfully reproducing the solar spectrum. Versions for agronomic lighting are also appearing on the market to take advantage of this high energy efficiency.
Most plasma lamp manufacturers use the principles of the first generation plasma lamps.
Modern plasma lamp
In 2012, the second generation of plasma lamps was born. This second generation eliminates a number of technical limitations of the first generation while retaining the advantages of the first generation. The main difference is in the bulbs, the first generation required rotating bulbs, while the second generation used static bulbs. By eliminating the motor used to rotate the bulb, this technological leap makes the system more compact and significantly reduces failure rates.
How does the plasma lamp work?
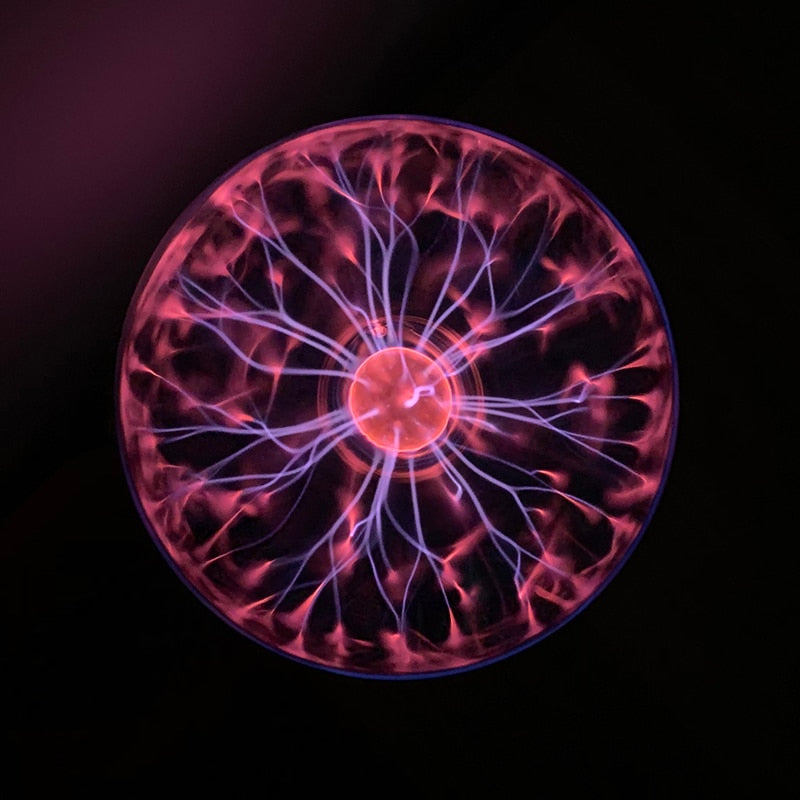
The plasma lamp consists of an electrode that represents a light bulb. Its frequency is constant and varies from 10 kHz to 60 kHz. Inside the glass ball, there is a mixture of noble gases under low pressure. These gases can be oxygen, neon, nitrogen, xenon, helium, krypton etc.. They are responsible for the color of the electric filaments.
They actually come in different colors. In the case of helium, it is blue-violet or yellow-orange. If the gas it contains is neon, the color of the flashes is red-orange. If it is krypton, the color is gray or yellow. In the case of xenon, it is blue, in the case of nitrogen, it is purple, in the case of carbon dioxide it is white. As for oxygen, it produces a light blue-green color.
There are also differences in color if the combination of different gases changes, here are some examples: Neon (95%) and Xenon (5%): pink and blue Neon (95%), xenon (2.5%) and krypton (2.5%): green and orange Argon and nitrogen: purple Argon, Nitrogen and Krypton: lavender purple, with blue tips Helium and neon: red and orange Xenon, krypton and argon: navy blue Neon (99.5%) and Argon (0.5%): purple and orange.
The operation of a plasma lamp is not complicated. Indeed, the central electrode will ionize the gas because of the applied tension. This phenomenon results in an electric discharge between the electrodes and the wall of the sphere.
If the applied voltage is high enough, it can overcome the breakdown voltage of the gas. As a result, an arc begins to form between the center of the lamp and the glass ball. This arc occurs in the form of small colored flashes.
The gas becomes a conductive plasma. This creates an electrical chain reaction. These responses are intensified if you touch the plasma light with your fingers.