Crystals are everywhere. You can find them in nature, in your tools or even in your kitchen. But, have you ever wondered how these materials are formed? Crystals are formed when chemical molecules line up in an orderly fashion to create a structure with repeating patterns. This is the same way snowflakes are formed! When water freezes, it causes these ordered structures to form. The unique patterns of each crystal depend on many factors such as temperature and humidity at the time of formation. Learn more about crystal formation.
What is a crystal ?
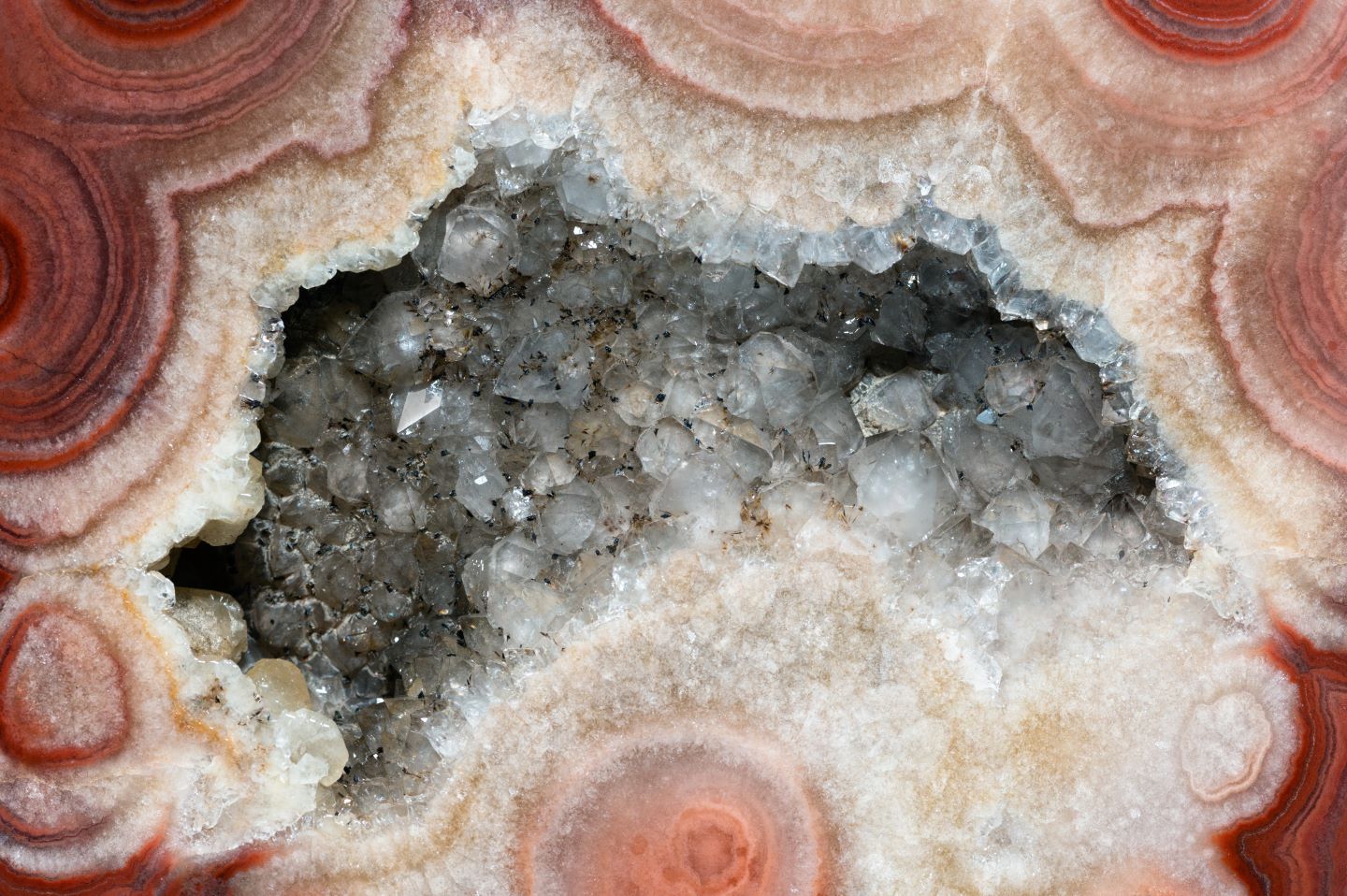
Crystals are materials that have a very regular and repetitive arrangement of their atoms or molecules. It is this ordered pattern that makes crystals so special! The atoms in a crystal are usually arranged in a three-dimensional grid, which gives crystals their characteristic sparkling appearance.
Crystals are formed from solutions, which means that the chemicals and molecules that make them up are mixed together in liquid form. When these molecules are slowly cooled, they begin to align themselves in an orderly fashion. The slower the molecules cool, the larger and more perfect the crystals.
The stages of crystal formation
-
Creation of a nucleus
The first step in the formation of a crystal is the creation of a nucleus. This can be done randomly, but crystals will form more easily if you provide a small seed to start with. The nucleus can be formed by any number of things, including dust and ice particles in the air, or even a single speck of dust on your fingers!
-
Crystallization
Crystallization refers to the process by which molecules line up to form a crystal. This happens when the nucleus is surrounded by a solution that contains the right molecules. The molecules in the solution will attach to the nucleus and begin to form a crystal.
-
Crystal growth
As the crystal grows, more and more molecules are deposited into its structure. The rate of growth is determined by various parameters, including the temperature and humidity of the solution.
The different types of crystals according to the crystallization process
Crystallization produces many different types of crystals with unique properties! The shape and pattern produced by each crystal depends on a number of factors, such as the temperature at the time of formation.
There are several names for the shapes that occur in crystals, including the six main shapes below:
Cubic: this is the most common shape for a crystal, with six square faces that meet at right angles
Octahedral: This shape has eight triangular faces and is a common shape in crystals with high symmetry
Triclinic: This is an irregular crystal with three main axes, which allows two different shapes at the same time!
Monoclinic: This crystal has only one axis of symmetry. It contains unequal sides that meet at angles other than 90 degrees and is recognized by its oblique sides
Orthorhombic: This crystal has three axes of symmetry and right angles between them. The shapes are rectangular
Hexagonal: This is a six-sided crystal with two axes of symmetry passing through the center. It is recognized by its curved edges.
The different types of crystals
Crystals found in nature
Crystals are not always pure, which makes it difficult to determine their exact composition. Here are some examples of the most common minerals found in nature:
Fluorite (CaF₄) is a mineral that contains calcium fluoride and hydrofluoric acid (HF). It has long been used as a source of fluorine, which is a key ingredient in toothpaste and other products.
Quartz (SiO₂) is the most common mineral on Earth, making up about 12% of the planet's crust. It is found in many different forms, including quartzite and amethyst.
Calcite (CaCO₃) is a mineral most commonly found in limestone and marble. It is a key ingredient in cement and is used to make plaster of Paris.
Crystals grown in the laboratory
In the laboratory, we can create crystals of any shape and size by controlling the conditions under which they form. This gives us great flexibility in terms of what we can do with these materials! Here are some common applications of synthetic crystals:
- Semiconductors: these are materials used in electronic devices to control the power of light or carry signals.
- Catalysts: these are substances that change the rate of chemical reactions, making them more efficient and less expensive. They allow us to create new materials used in a wide range of applications, including pharmaceuticals and automobile engines!
- Diamond crystals: they can be used as abrasives or cutting tools due to their hardness. They are also used to make drills and the tips of some needles!
- Glasses: these are materials that can be formed by a process similar to crystallization, but in which the atoms do not assemble into crystals. Glasses have many applications, including windows in buildings and cars, lenses in cameras, and fiber optics that allow for quality images on the internet.
- Semiconductor lasers: these are devices that contain a semiconductor, which allows for a high-energy laser light beam with low energy consumption! This type of laser can be found in CD or DVD players, as well as in barcode scanners in supermarkets, gas stations and many other places !




Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.